Update: Comment Period Ends – AATB Response to FDA Draft Guidance Document Sepsis
Thanks for joining! As the comment period has closed, let’s dive into the American Association of Tissue Banks (AATB) recommendations for the Food and Drug Administration’s (FDA) draft guidance document, “Recommendations to Reduce the Risk of Transmission of Disease Agents Associated with Sepsis by Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps)” [1] . The initial release of this document in January 2025 as a “final” guidance document caused major industry concerns leading to tissue bank stakeholders voicing their concerns to the FDA.
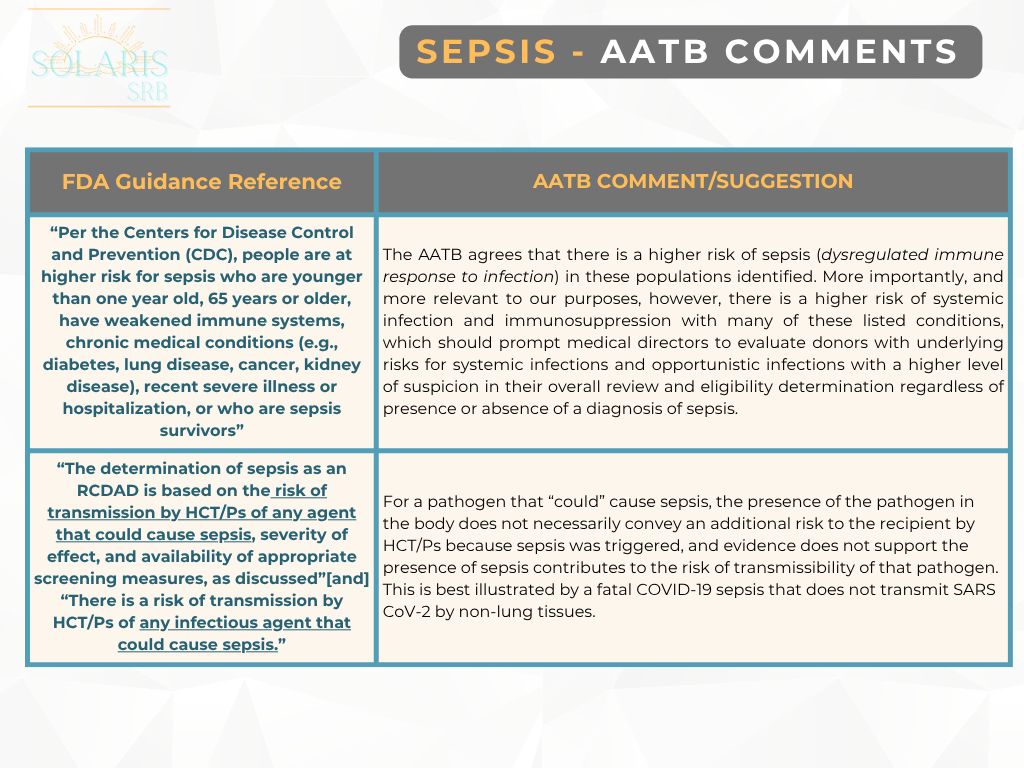
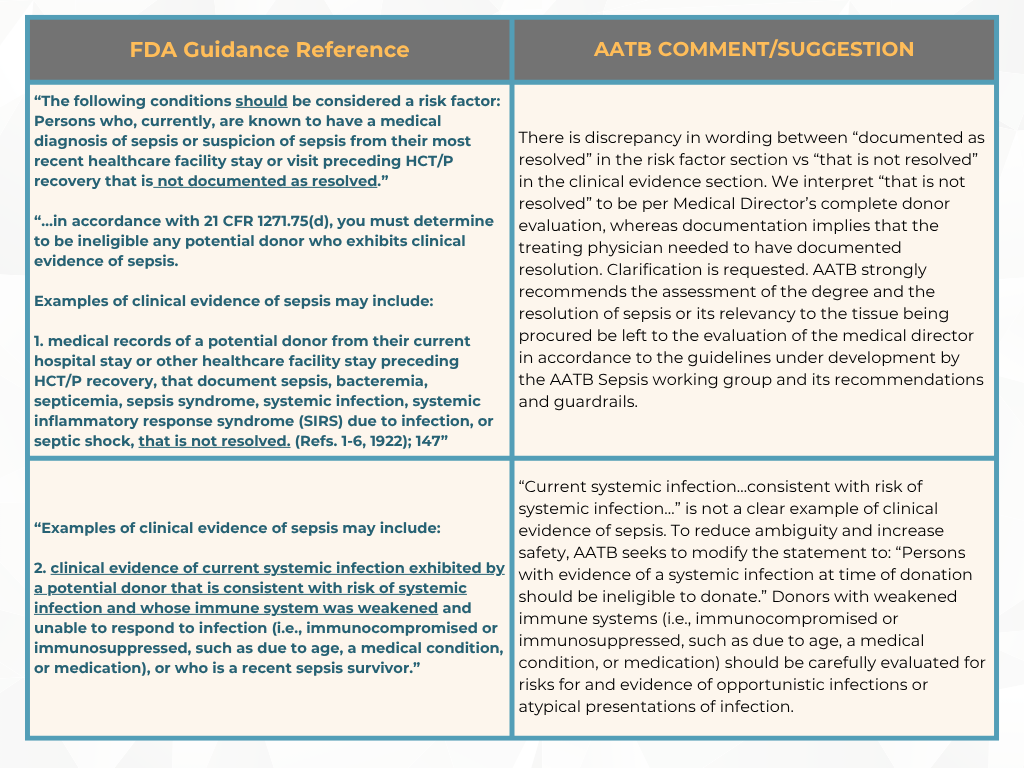
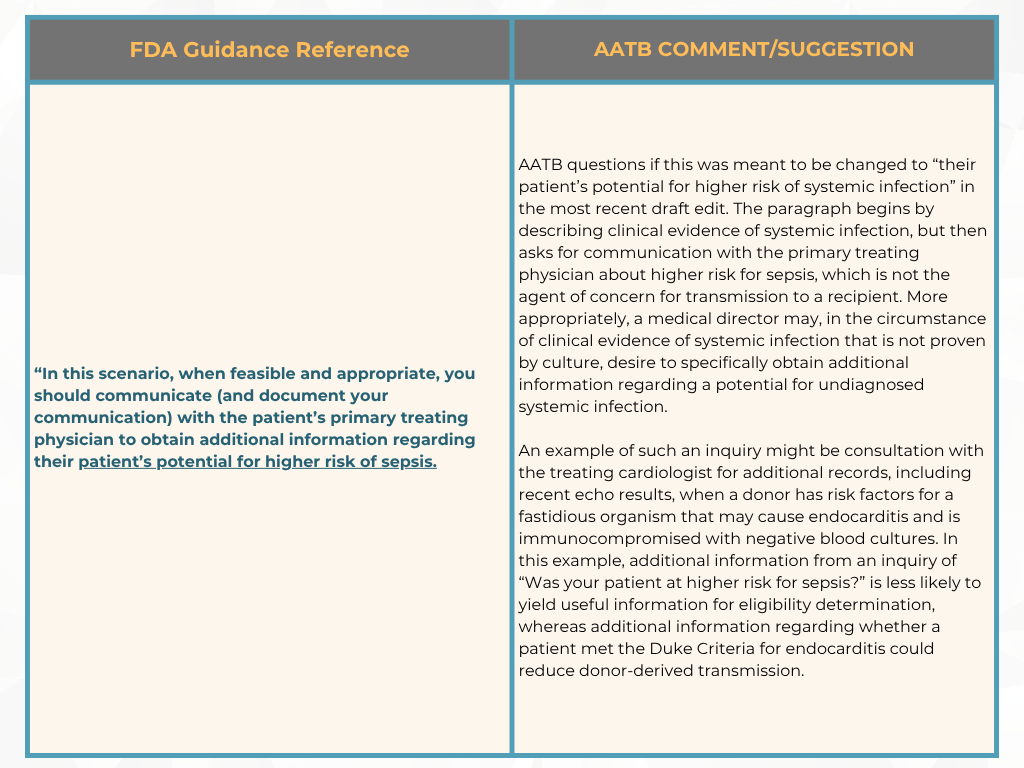
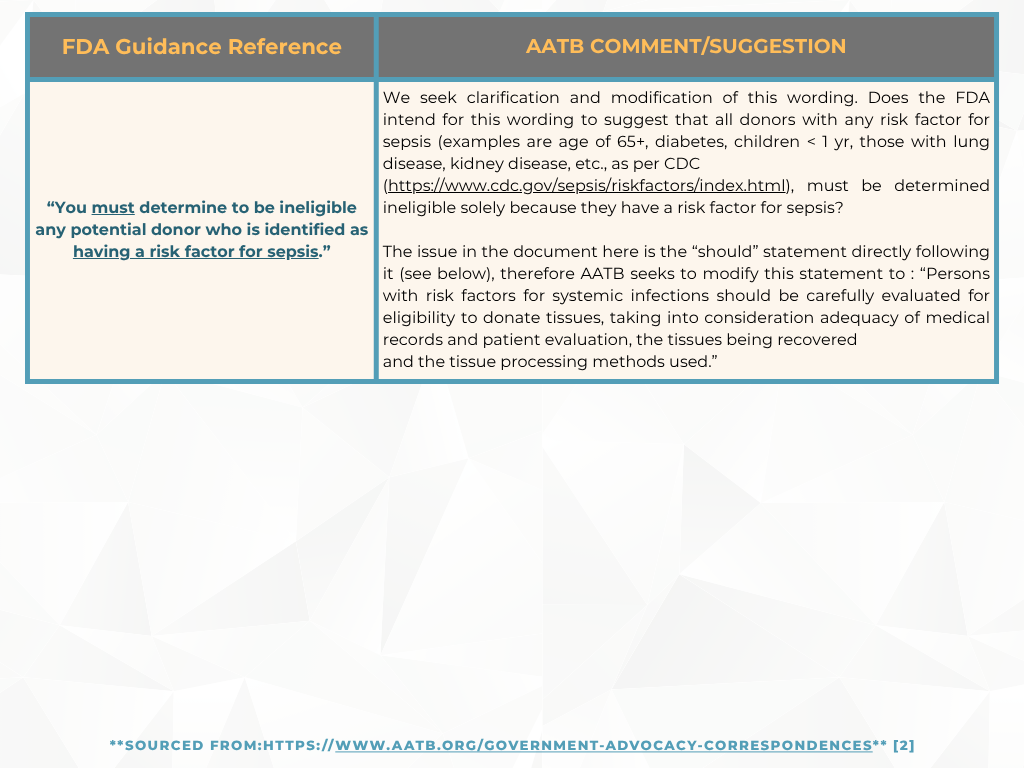
In response, the FDA withdrew the “final” guidance document and reissued it as a “draft” to include the public’s comments. From the original guidance document in August 2007, “Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps),” FDA classified sepsis as a relevant communicable disease agent or disease (RCDAD) and based on the current draft document, the AATB’s perspective suggests that this did not conform with the actual definition of RCDAD by FDA.
Previously, in May, we provided an update on the AATB’s response to the FDA and now with the comment period closed, here are a few areas the AATB has submitted for additional clarification [2]
Concerns with the Classification of Sepsis as an RCDAD
While FDA designated sepsis as an RCDAD in 2007 to help identify at-risk tissue donors, using a clinical diagnosis of sepsis as a stand-in for transmissible infectious disease is not scientifically justified. The key issue should be whether a donor’s underlying infection could pose a risk to recipients, not just the presence of sepsis. Relying on a sepsis diagnosis may encourage automatic donor deferral rather than thoughtful evaluation, potentially leading to unnecessary deferral of donors who don’t actually pose an infectious risk.
Sepsis Does Not Meet FDA’s Conditions for RCDADs
FDA’s definition for RCDADs include three (3) characteristics: 1) Risk of transmission, 2) Severity of effect, and 3) Availability of appropriate screening measures or tests.
Risk of transmission – AATB’s thoughts are that the infection (disease agent) is what conveys the risk associated with tissue product, not the potential result of sepsis (as in it is not possible to transmit a dysregulated host response to infection). Therefore, the risk of transmission should be determined based upon the infectious cause of the sepsis rather than the diagnosis of sepsis alone.
Severity of effect – AATB states that sepsis is not a transmissible disease, therefore sepsis itself does not result in risk to the recipient. Sepsis is a “host specific/personal” chemical and physiologic dysregulated immune response and cannot be transmitted. The dysregulated response itself is not transmissible.
Availability of appropriate screening and/or testing measures – AATB’s thoughts are that clinically, screening for sepsis is a pre-mortem prognostic tool used to predict mortality and enables implementation of rapid preventative measures, not a diagnostic tool for detecting infection.
Changing Practices in US Healthcare and Clinical Considerations
Different definitions of sepsis are in use today which presents a challenge and are driven by hospital coding and billing practices for billing incentives. Clinicians including sepsis in a differential diagnosis may occur to ensure timely life-saving treatments, but the subsequent diagnosis reveals that the physiological manifestations initially attributed to sepsis may be due to non-infectious etiologies, and therefore, the presence of a sepsis diagnosis may not reliably indicate an underlying transmissible infectious disease.
Here are additional AATB comments proposed:
For additional information and the full details, check out AATB’s submission here!
Keep checking back as we keep up with how this final guidance evolves!
References:
[1] https://www.fda.gov/regulatory-information/search-fda-guidance-documents/recommendations-reduce-risk-transmission-disease-agents-associated-sepsis-human-cells-tissues-and
[2] https://www.aatb.org/government-advocacy-correspondences