Unique Device Identifier (UDI) Requirements for Combination Products
Welcome back! Today we’re diving into the Food and Drug Administration’s (FDA) recent draft guidance, “Unique Device Identifier (UDI) Requirements for Combination Products”, currently in its comment period from June 26, 2025 [1] . This guidance represents significant progression in the medical device (“devices”) industry regulation in protecting patient safety. This system aims to create a standard by assigning unique identifiers to devices including their labels and packaging, while enhancing traceability, surveillance, and management through the medical device’s lifecycle.
The objectives of the UDI system address the following:
Improve patient safety: by making medical devices easier to identify, device information is more quickly and accurately retrievable which reduces the risk of medical errors.
Recall efficiency: when defective or unsafe medical devices are identified, the UDI system enables faster identification and removal of these products from circulation.
Enhanced post-market surveillance: this system enables regulators, manufacturers, and healthcare providers to more effectively monitor device performance in real world settings supporting data-driven decisions.
Streamlining supply chain management: the standardized UDIs make it easier to manage inventories, track device movement, and authenticate products.
What makes up the UDI system [2] ?
Unique Device Identifier (UDI) – A UDI is a series of numeric or alphanumeric characters that is created through an internationally accepted device identification and coding standard. Each UDI consists of two segments—the Device Identifier (DI) and the Production Identifier (PI).
Device Identifier (DI) – The DI is the mandatory, fixed portion of a UDI that identifies the specific version or model of a device and the labeler of that device.
Production Identifier (PI) – The PI is the conditional, variable portion of a UDI, that identifies one or more of the following when included on the label of a device:
Lot or batch number within which a device was manufactured
Serial number of a specific device
Expiration date of a specific device
Date a specific device was manufactured
Distinct identification code required by 1271.290(c) for a human cell, tissue, or cellular and tissue-based product (HCT/P) regulated as a device
including production-related data such as the device’s lot or batch number, serial number, manufacturing date, or expiration date.
Labeling Requirements – Manufacturers must include the UDI in both plain text and machine-readable (AIDC—Automatic Identification and Data Capture) formats on device labels and packages. Some devices must also have the UDI marked directly on the device itself (direct marking), especially if they are intended for multiple uses and are reprocessed between uses.
Global UDI Database (GUDID): The FDA established the GUDID database to serve as a central repository for key device identification information associated with each UDI. Labelers are responsible for submitting and maintaining accurate device information in the GUDID.
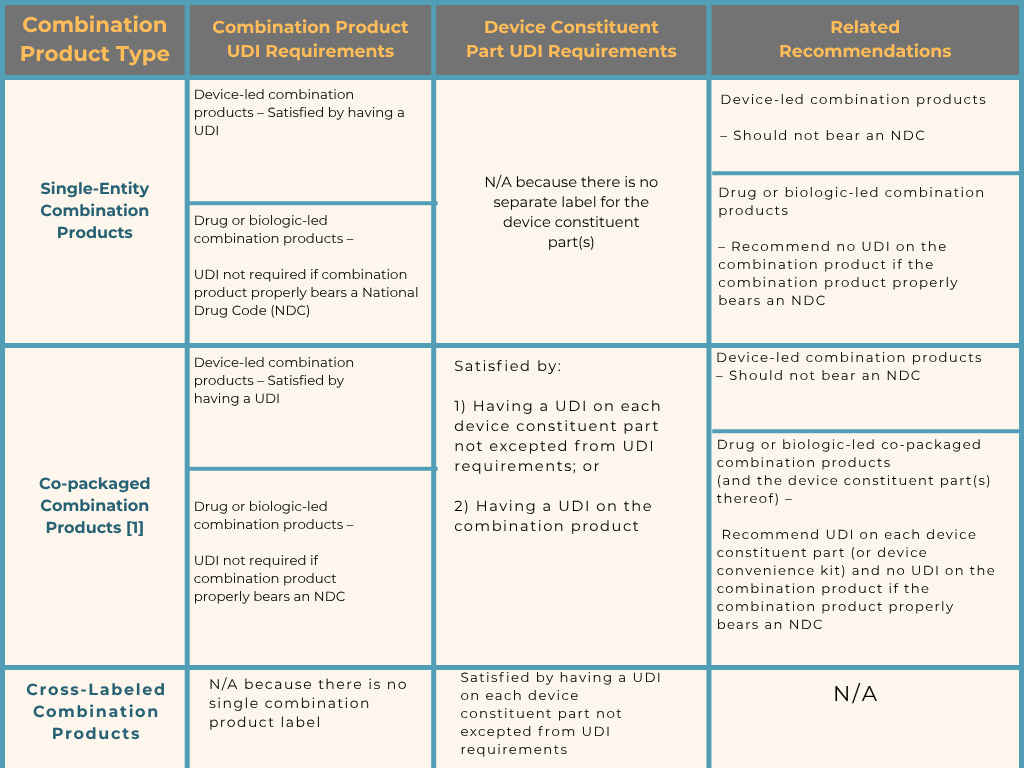
Combination products integrate drugs, devices, and/or biological products (e.g., drug/device, biologic/device, drug/biologic, or all three) and are unique due to their ‘blended’ nature. Each drug, device, and biological product part of a combo product is called the “constituent part”. Not all combination (“combo”) products are subject to UDI labeling requirements such as those that may be primarily regulated as a drug or biologic, instead, the FDA looks at the “device constituent part” of the combo product to determine if it falls in scope. Check out the summary of FDA’s thoughts in the table below:
*This table assumes the combination product contains at least one device constituent part that does not fall into an exception at 21 CFR 801.30(a)(1) - (10), see section III.A, and assumes the use of unique device identifier (UDI) instead of universal product code (UPC)[ 1] .
Check back later as we continue following the FDA, once they’ve gathered more information from the public’s comment period, we look forward to the final guidance to come!
Do you have questions about combination products and how this could impact your scope? Reach out to us with questions here: Contact Us!
See you next time!
References:
[2] https://www.fda.gov/medical-devices/unique-device-identification-system-udi-system/udi-basics