Chorioamnionitis in Birth Tissue Donation
Have you heard of Chorioamnionitis? Today we’re going to dive in and provide an overview of why this infection poses challenges to the birth tissue donation industry. With the use of birth tissues in regenerative medicine and transplantation, ensuring the safety and quality of donated tissues is paramount. Let’s go!
Chorioamnionitis is a serious infection that affects a mother during pregnancy. It gets its name from the two membranes that surround a fetus in the uterus: the chorion and the amnion [1] . Chorioamnionitis is an infection that can occur before or during labor, or after delivery. It can be acute, subacute, or chronic. It is associated with chronic lung disease in the infant. Chronic chorioamnionitis is associated with retinopathy of prematurity, very low birth weight, and impaired brain development in the premature infant. Chronic chorioamnionitis is also common. It’s most commonly associated with preterm labor, prolonged rupture of membranes, prolonged labor, tobacco use, nulliparous pregnancy, meconium-stained fluid, multiple vaginal exams post rupture of membranes, and in women with known bacterial or viral infections. However, it can occur at term and in women without prior infections. Chorioamnionitis can lead to morbidity and mortality for the mother and neonate if left untreated. Antibiotic therapy has been shown to reduce the incidence and severity of the infection in both the mother and neonate [2].
The presence of chorioamnionitis in birth tissues significantly increases the risk of microbial contamination. Transplantation of infected tissues can result in serious complications for recipients, including transmission of infection, graft failure, inflammatory responses, and delayed healing. Those who are immunocompromised will be particularly vulnerable to adverse outcomes. Therefore, rigorous screening and exclusion of tissues from donors with chorioamnionitis is essential to protect transplant recipients and uphold the standards of tissue banking.
Screening for chorioamnionitis involves a combination of items but not limited to:
Medical history and record review must be performed for risk factors such as prolonged labor, premature rupture of membranes, and intrapartum fever.
Clinical assessments of maternal vital signs, uterine tenderness, and amniotic fluid characteristics are a few that should be reviewed.
Review of laboratory test results such as white blood cell count, C-reactive protein [3] (also known as CRP, a protein made by the liver, the level of CRP increases when there’s inflammation in the body), and microbiological cultures from the placenta or membranes when indicated, may be helpful in identifying.
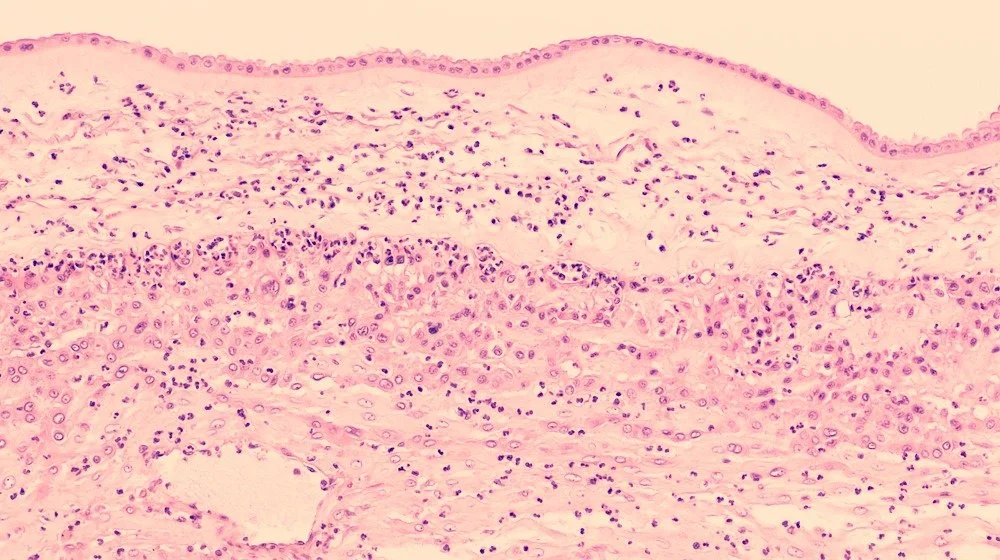
Histological evaluation of placental tissues for evidence of acute inflammation may help from a pathological examination perspective.
Criteria for acceptance and deferral are established to ensure only safe, high-quality birth tissues are processed and distributed based on Medical Director discretion regarding pathology and risk. Considerations should include, but not be limited to:
Automatic deferral for donors with a clinical diagnosis of chorioamnionitis, intraamniotic infection, or maternal fever during labor.
Tissues with histological evidence of acute inflammation should be considered for exclusion.
Adherence to standards set by the FDA and AATB which mandate exclusion of tissues from donors with infectious risks.
Acceptance for donors with no evidence of infection and all tissues pass rigorous safety checks before being released for transplantation.
Best practices play a critical role in safeguarding recipient health through robust screening and quality practices, key considerations include:
Training staff involved in donor screening and tissue acquisition to recognize signs of chorioamnionitis.
Adopt evidence-based protocols for donor assessment and tissue evaluation.
Regular review of screening outcomes and adverse event reports to identify areas for improvement.
Compliance with external audits and accreditation processes to ensure ongoing adherence to safety standards.
Effective documentation and communication between clinicians, tissue banks, and regulatory authorities are essential to ensure the traceability and safety of all donated birth tissues.
Chorioamnionitis represents a significant risk factor in the birth tissue banking industry, with the potential to compromise transplant outcomes and recipient safety. Rigorous screening, strict acceptance and deferral criteria, and adherence to regulatory standards are essential to mitigate these risks. Ongoing education, documentation, and quality practices further enhance the safety and reliability of birth tissue transplantation.
Thanks for stopping by, keeping checking back as we continue following industry trends!
Have any questions? Reach out to us here!
References
[1] https://my.clevelandclinic.org/health/diseases/12309-chorioamnionitis
[2] https://www.ncbi.nlm.nih.gov/books/NBK532251/
[3] https://www.mayoclinic.org/tests-procedures/c-reactive-protein-test/about/pac-20385228